Experiment VIII, Leoben, 17 September 2021
For this set of experiments, Ernst Logar, Michael Hohenberger and Karez Abdulhameed visited the Chair of Thermal Processing Technology at Montanuniversität Leoben. The focus of the experiments was on the flammability of crude oil and the calculation of related parameters.
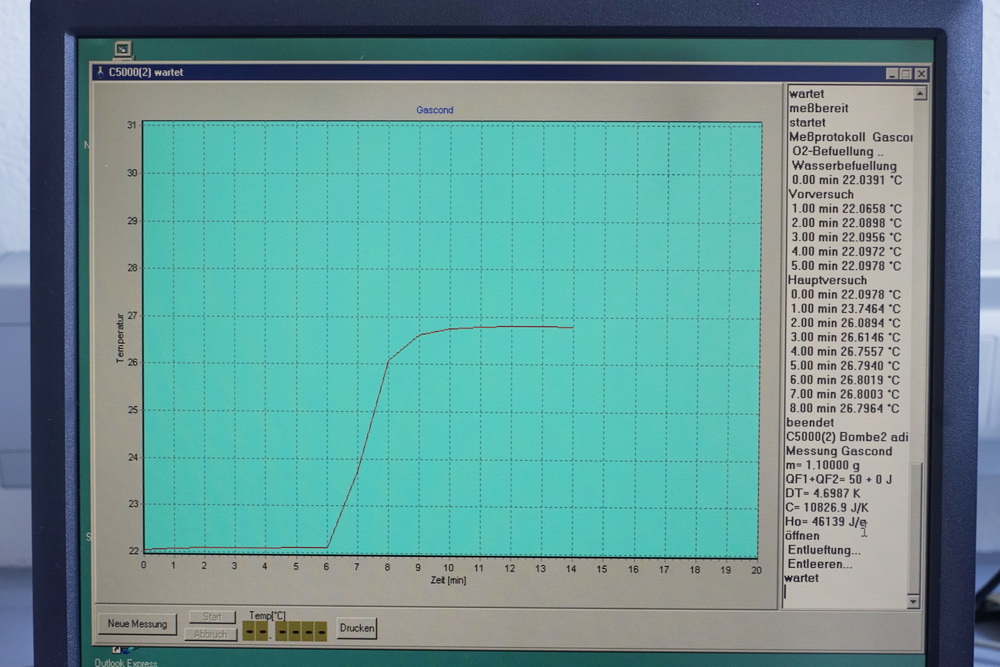
The aim of their first experiment was to find out how many calories different samples of crude oil contain. For this they used a bomb calorimeter, crude oil samples and igniting threads. One gram of crude oil was weighed into the sample cup of the calorimeter, the igniting thread then wrapped around the electric wire and immersed in the crude oil. The closed cup was then placed into the bomb calorimeter. The bomb calorimeter measures the heat of combustion. Electrical energy is used to ignite the fuel, and as it burns the surrounding air heats up, expands, and escapes through a tube, heating the water surrounding the tube. The change in the temperature of the water allows us to calculate the caloric content of the fuel. The bomb calorimeter device’s software renders the results as graph patterns.
The second experiment sought to create ink for painting purposes by burning crude oil to then collect the soot from the flames. For this, a heat resistant ceramic bowl, glass tube, and acetone were used. A small quantity of crude oil was poured into the bowl and a continuous source of fire introduced until the oil would start burning by itself. However, the first sample stopped burning when the fire source was removed. In the second attempt, a benzoic acid tablet was crushed and added to the crude oil. This time the crude oil burned longer, but needed to be reignited several times. The reason for this is that heavier mixtures of crude oil compounds are less easily flammable than the distilled fraction of the crude oil or lighter compounds.
Afterwards a small piece of paper was placed in the crude oil and set on fire. This time, with the paper, the oil burnt for the longest period. Soot was then collected by washing the inner tube with acetone into a container, after an attempt to collect the soot using filter paper failed. Very little light grey soot could be collected from the first two tries. There was more soot left after the last experiment set-up and it was darker because of the carbon present in the paper.
In the third and last experiment, a “crude oil fire cake” was created to investigate the explosion temperature of crude oil and the maximum amount of soot that can be produced from a certain quantity of oil. For this a high temperature oven was used. A small amount of crude oil was placed in a heat resistant container and placed in the oven at 700 degrees Celsius. Dark smoke started to come out of the oven’s ventilator tube almost immediately. When the oven door was opened less than a minute later, a big dark flame shot out. The temperature was reduced, and the oil sample was kept in the closed oven until the smoke coming out of the ventilator tube slowly dissipated. The crude oil sample was completely burned up, leaving a very sticky layer at the bottom of the container.
Chair of Thermal Processing Technology I flammability I bomb calorimeter I caloric value I ink I soot I paper I carbon I smoke